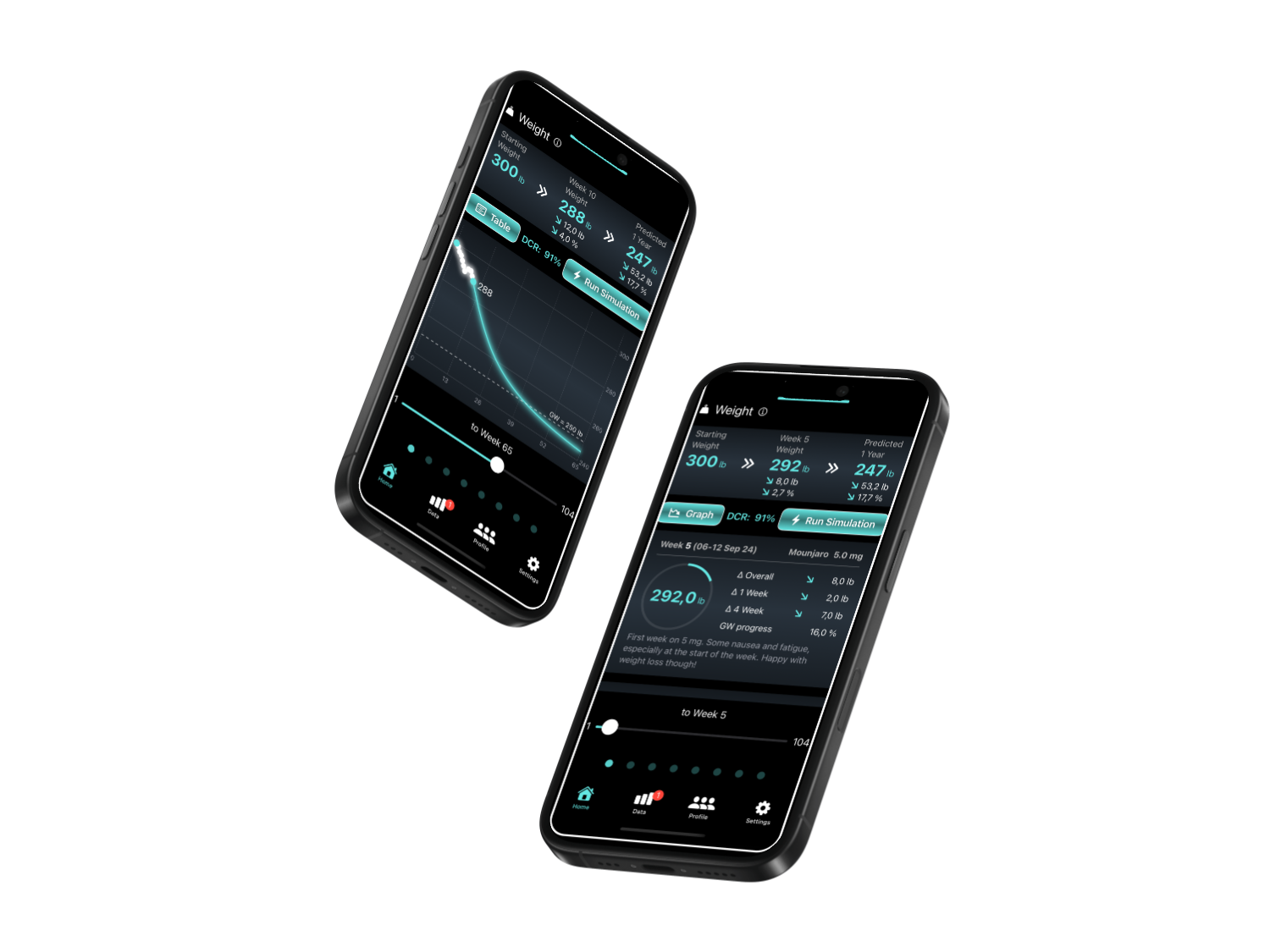
Ready to track, predict and compare your weight loss journey with Wegovy, Zepbound, Ozempic or Mounjaro?
Simple to Use
Select your drug, and then capture your data; only 1 minute each week!
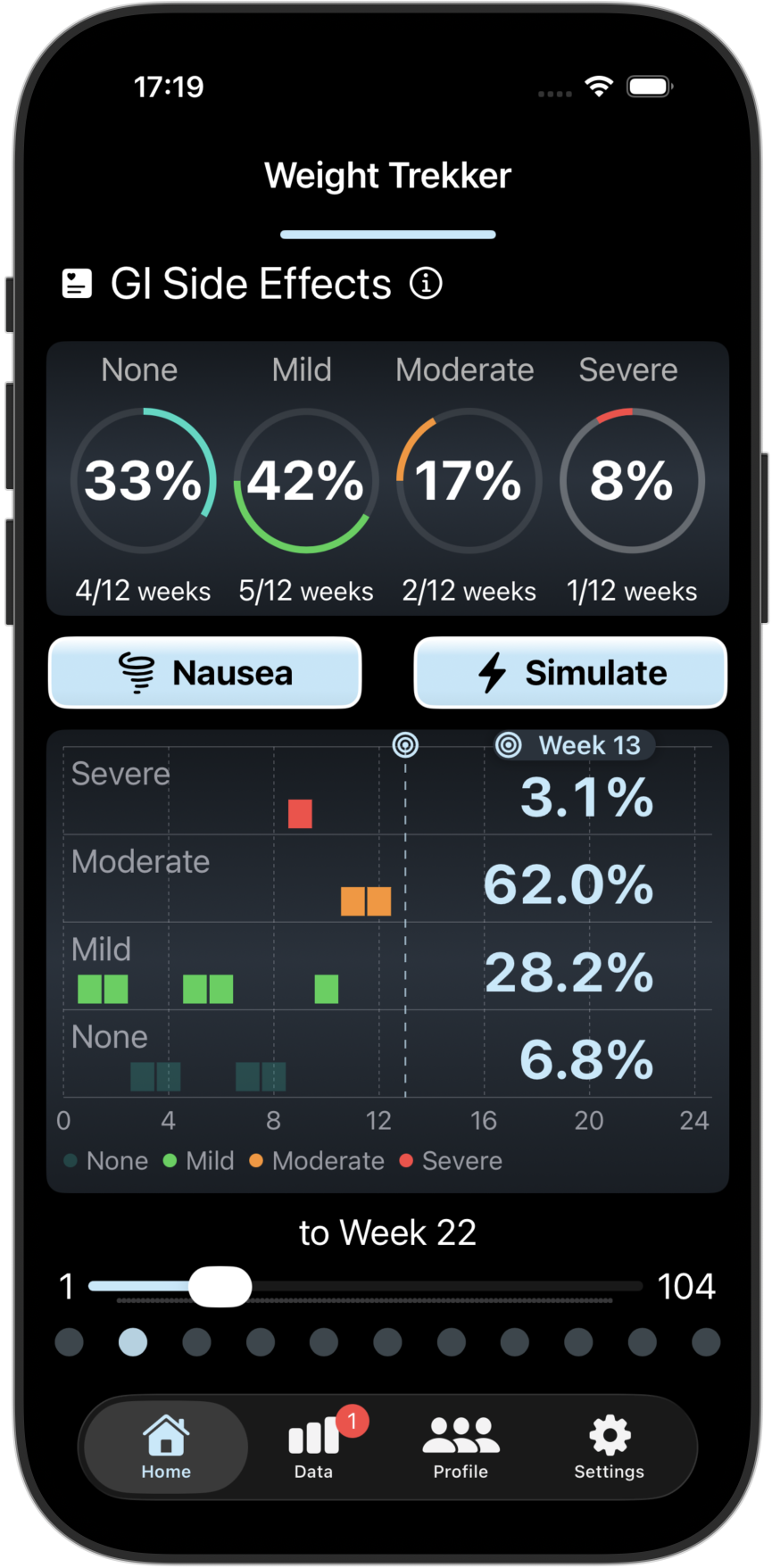
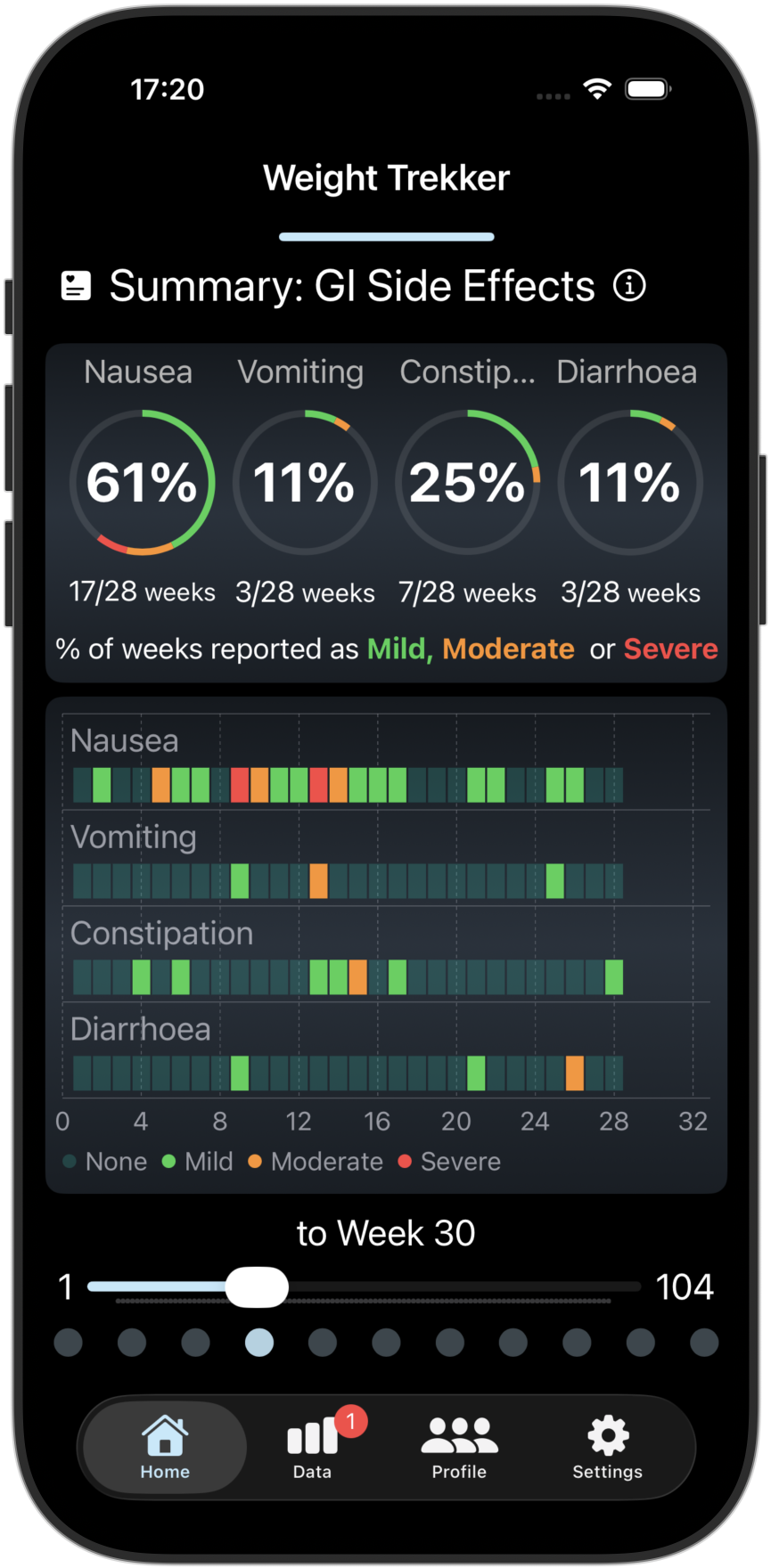
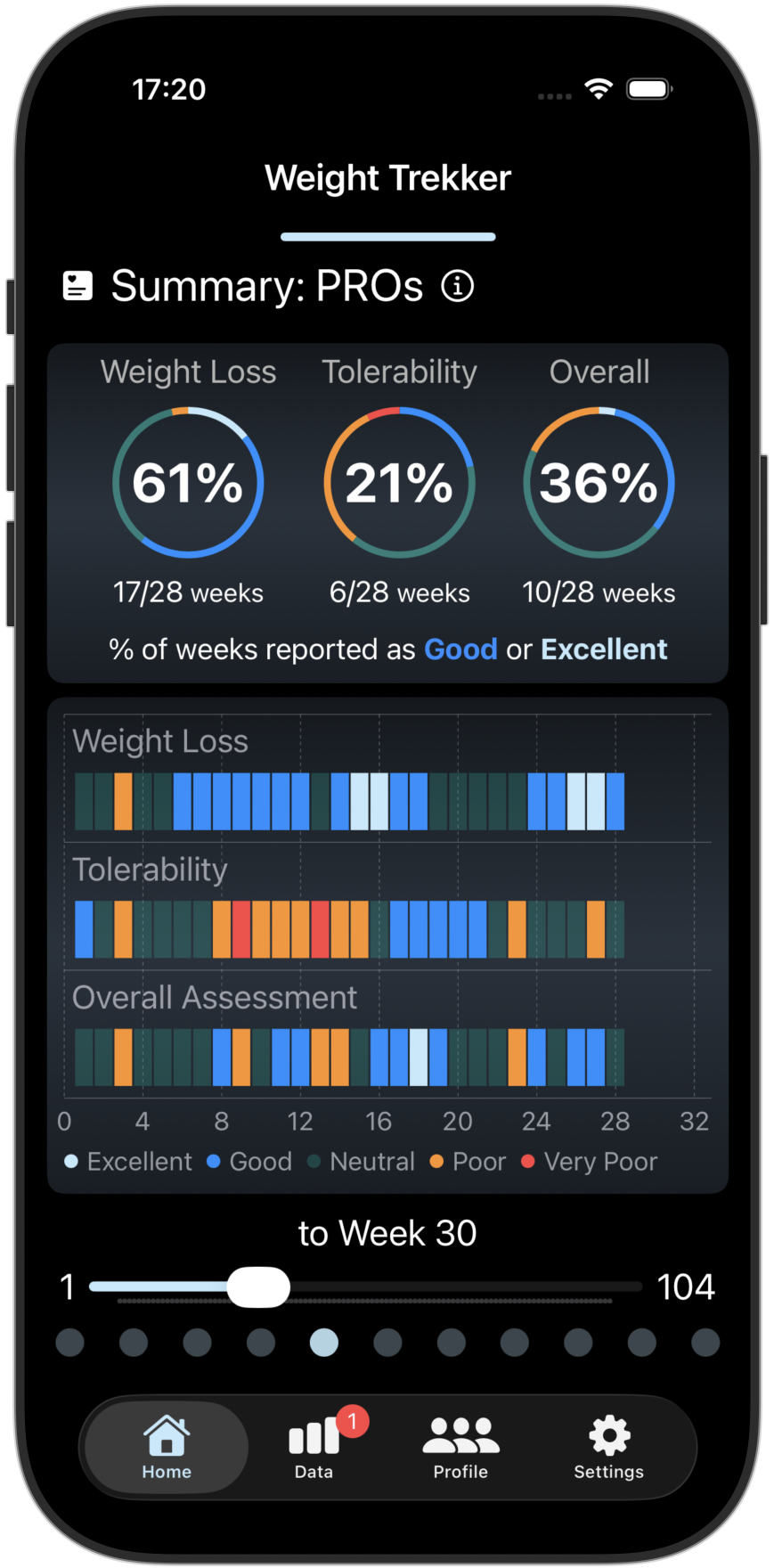
Track/Compare your GI Side Effects and PROs
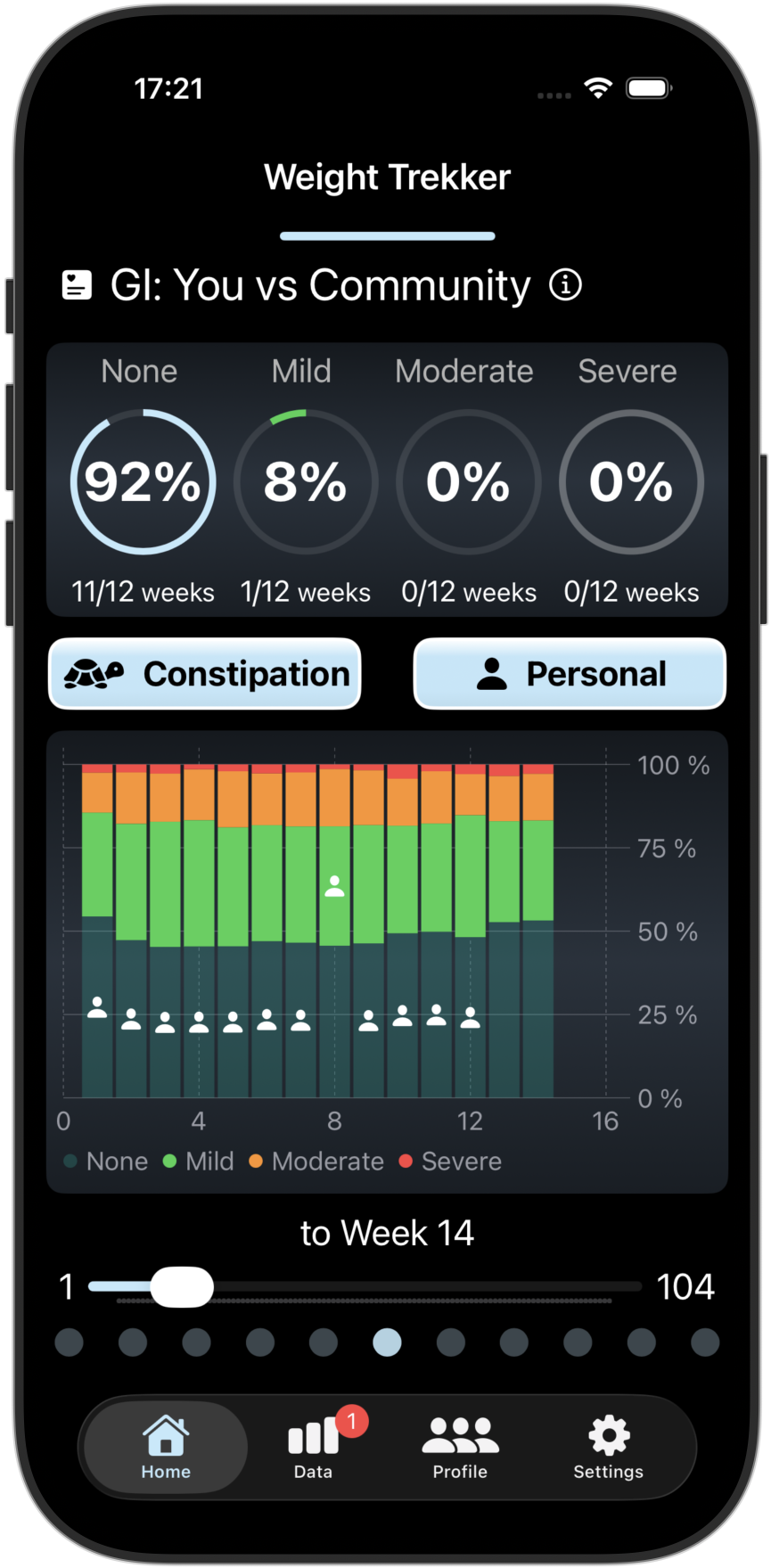
Tracks 4 key GI Side Effects and 3 Patient Reported Outcomes (PROs). Compare your results with other GLP-1 users.
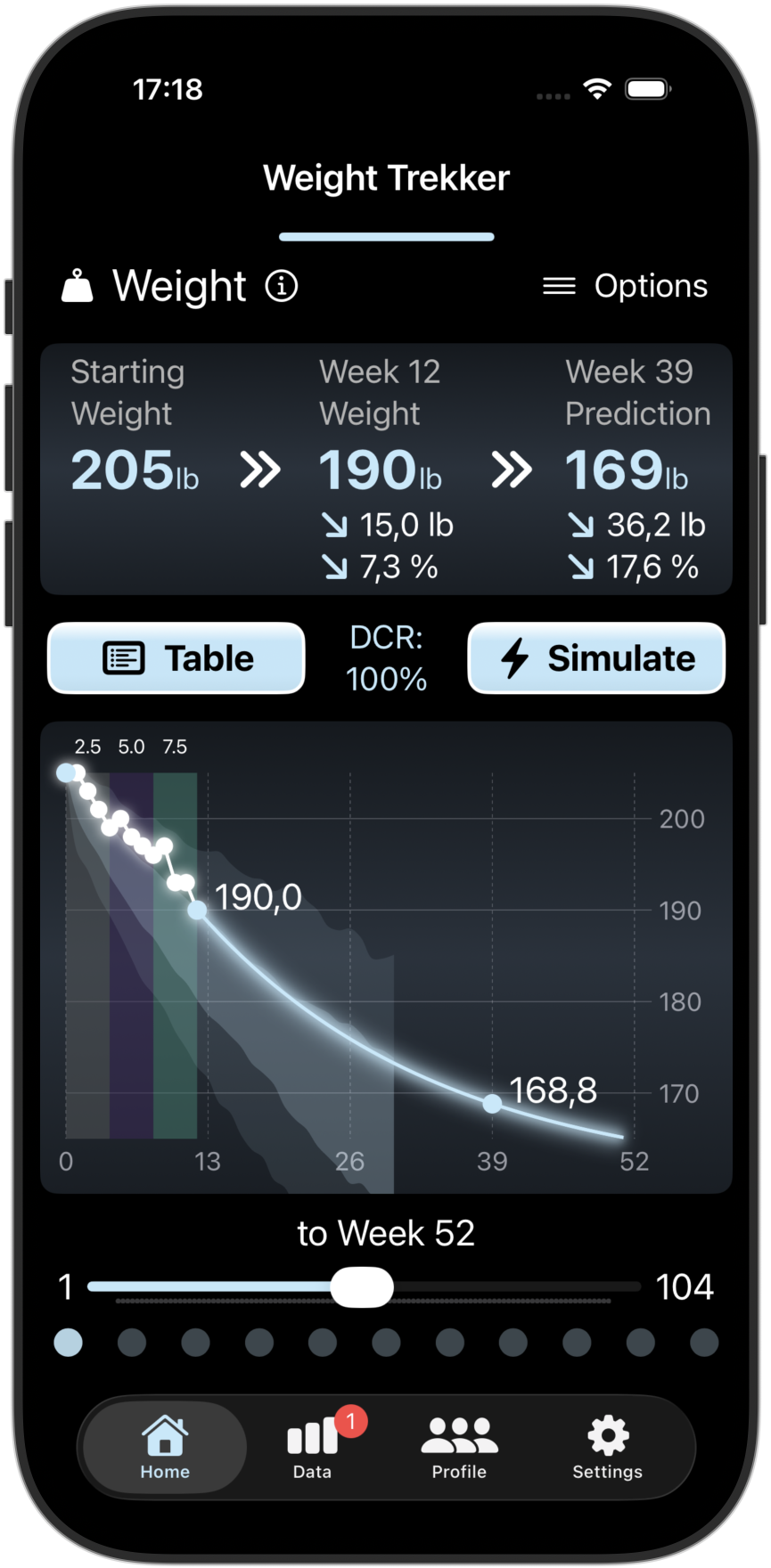
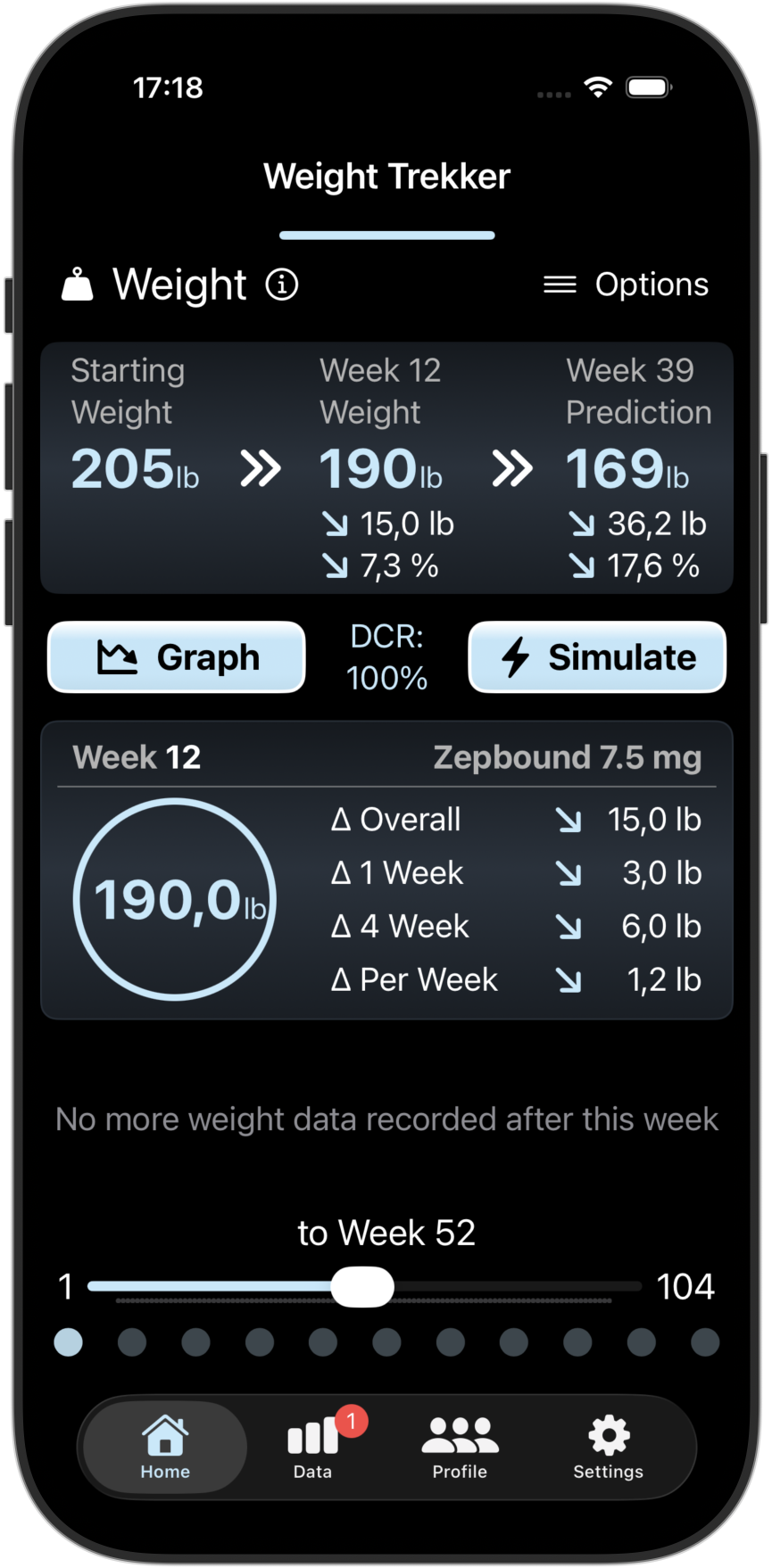
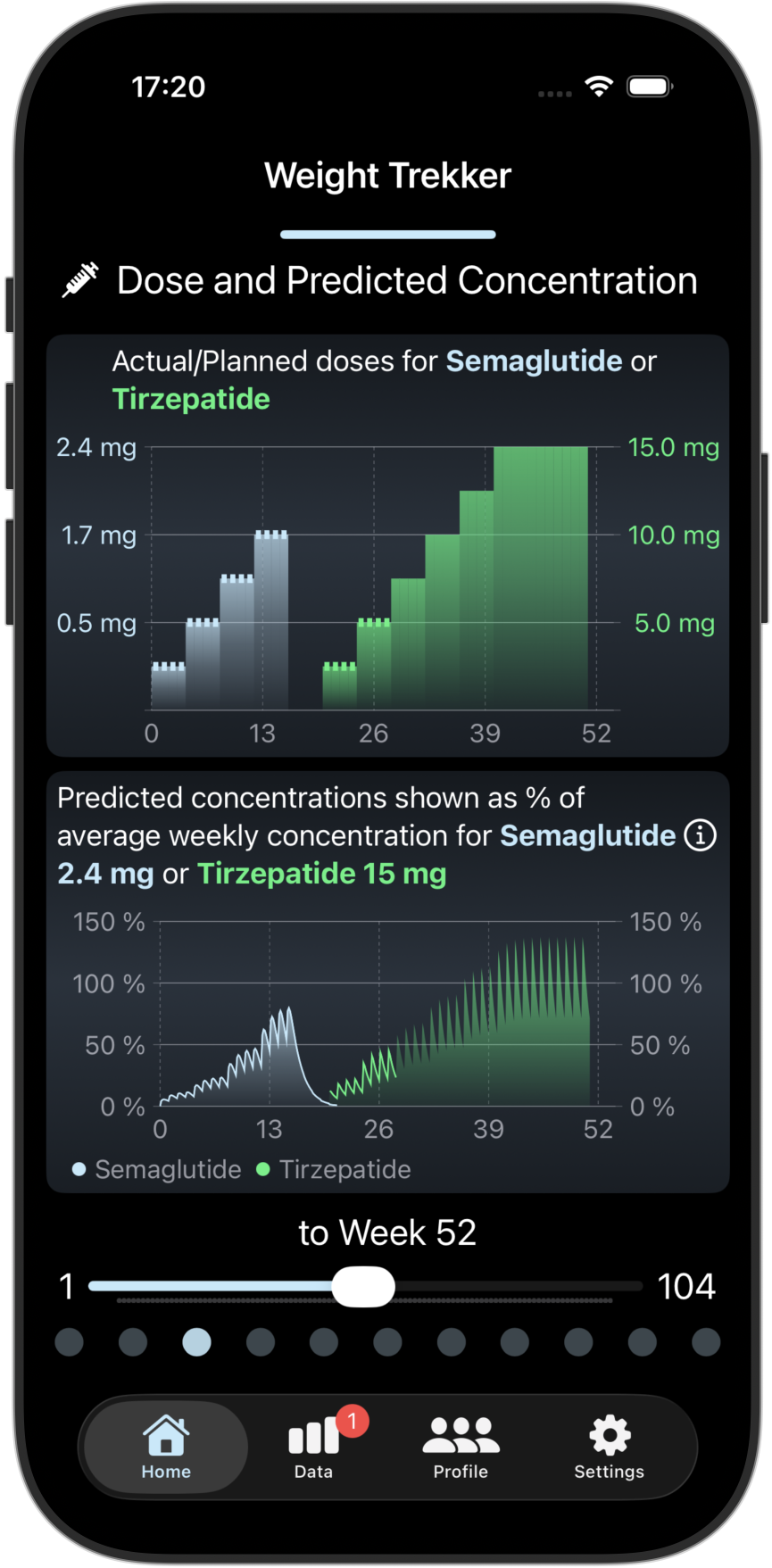
Scientific Predictions
Our advanced PKPD prediction models uses your previous/planned doses and your observed weight changes to predict your future weight changes and predicted GI risks in the coming week. You can also see how your progress compares with others.
Screenshots from the Weight Trekker App

Frequently Asked Questions
The app is designed to work on all modern iPhones and iPads.
Yes. The App has been developed in a wholly independent manner, with no funding or grants from any company or organisation. Our community results are the actual, honest results from other GLP-1 users. Weight Predictions are based on an integrated analysis of the clinical trial programs for semaglutide (SUSTAIN and STEP) and tirzepatide (SURPASS and SURMOUNT), along with information in the FDA reviews for each drug.
If you have any problems, we are very happy to help. Please contact us at support@weighttrekker.com.
The app is designed to work on all modern iPhones and iPads.
We're sorry if you've encountered any issues while using our app. If you have noticed any bugs, errors or unusual behavior, please do kindly let us know by clicking the link below. Your feedback will help us fix errors and improve the app. Contact us at support@weighttrekker.com
Medical Sources
Pharmacokinetics for tirzepatide
The pharmacokinetics for tirzepatide use the pharmacokinetic model developed and presented in the following publication.
Schneck, Karen, and Shweta Urva. ‘Population Pharmacokinetics of the GIP/GLP Receptor Agonist Tirzepatide’. CPT: Pharmacometrics & Systems Pharmacology 13, no. 3 (March 2024): 494–503.
https://doi.org/10.1002/psp4.13099.
Pharmacokinetics for semaglutide
The pharmacokinetics for semaglutide use the pharmacokinetic model developed and presented in the following publication.
Overgaard, Rune V., Philip H. Delff, Kristin C. C. Petri, Thomas W. Anderson, Anne Flint, and Steen H. Ingwersen. ‘Population Pharmacokinetics of Semaglutide for Type 2 Diabetes’. Diabetes Therapy 10, no. 2 (April 2019): 649–62.
https://doi.org/10.1007/s13300-019-0581-y.
The relevant clinical trial results for tirzepatide for the treatment of obesity and/or type 2 diabetes
SURMOUNT 1 - Jastreboff et al.
Jastreboff, Ania M., Louis J. Aronne, Nadia N. Ahmad, Sean Wharton, Lisa Connery, Breno Alves, Arihiro Kiyosue, et al. ‘Tirzepatide Once Weekly for the Treatment of Obesity’. New England Journal of Medicine 387, no. 3 (21 July 2022): 205–16.
https://doi.org/10.1056/NEJMoa2206038.
SURMOUNT 2 - Garvey et al.
Garvey, W Timothy, Juan P Frias, Ania M Jastreboff, Carel W Le Roux, Naveed Sattar, Diego Aizenberg, Huzhang Mao, et al. ‘Tirzepatide Once Weekly for the Treatment of Obesity in People with Type 2 Diabetes (SURMOUNT-2): A Double-Blind, Randomised, Multicentre, Placebo-Controlled, Phase 3 Trial’. The Lancet 402, no. 10402 (August 2023): 613–26.
https://doi.org/10.1016/S0140-6736(23)01200-X.
SURPASS 1 - Rosenstock et al.
Rosenstock, Julio, Carol Wysham, Juan P Frías, Shizuka Kaneko, Clare J Lee, Laura Fernández Landó, Huzhang Mao, Xuewei Cui, Chrisanthi A Karanikas, and Vivian T Thieu. ‘Efficacy and Safety of a Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide in Patients with Type 2 Diabetes (SURPASS-1): A Double-Blind, Randomised, Phase 3 Trial’. The Lancet 398, no. 10295 (July 2021): 143–55.
https://doi.org/10.1016/S0140-6736(21)01324-6.
SURPASS 2 - Frias et al.
Frías, Juan P., Melanie J. Davies, Julio Rosenstock, Federico C. Pérez Manghi, Laura Fernández Landó, Brandon K. Bergman, Bing Liu, Xuewei Cui, and Katelyn Brown. ‘Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes’. New England Journal of Medicine 385, no. 6 (5 August 2021): 503–15.
https://doi.org/10.1056/NEJMoa2107519.
SURPASS 3 - Ludvig et al.
Ludvik, Bernhard, Francesco Giorgino, Esteban Jódar, Juan P Frias, Laura Fernández Landó, Katelyn Brown, Ross Bray, and Ángel Rodríguez. ‘Once-Weekly Tirzepatide versus Once-Daily Insulin Degludec as Add-on to Metformin with or without SGLT2 Inhibitors in Patients with Type 2 Diabetes (SURPASS-3): A Randomised, Open-Label, Parallel-Group, Phase 3 Trial’. The Lancet 398, no. 10300 (August 2021): 583–98.
https://doi.org/10.1016/S0140-6736(21)01443-4.
SURPASS 4 - Del Prato et al.
Del Prato, Stefano, Steven E Kahn, Imre Pavo, Govinda J Weerakkody, Zhengyu Yang, John Doupis, Diego Aizenberg, et al. ‘Tirzepatide versus Insulin Glargine in Type 2 Diabetes and Increased Cardiovascular Risk (SURPASS-4): A Randomised, Open-Label, Parallel-Group, Multicentre, Phase 3 Trial’. The Lancet 398, no. 10313 (November 2021): 1811–24.
https://doi.org/10.1016/S0140-6736(21)02188-7.
SURPASS 5 - Dahl et al.
Dahl, Dominik, Yukiko Onishi, Paul Norwood, Ruth Huh, Ross Bray, Hiren Patel, and Ángel Rodríguez. ‘Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial’. JAMA 327, no. 6 (8 February 2022): 534.
https://doi.org/10.1001/jama.2022.0078.
The relevant clinical trial results for semaglutide for the treatment of obesity and/or type 2 diabetes
STEP 1 - Wilding et al.
Wilding, John P.H., Rachel L. Batterham, Salvatore Calanna, Melanie Davies, Luc F. Van Gaal, Ildiko Lingvay, Barbara M. McGowan, et al. ‘Once-Weekly Semaglutide in Adults with Overweight or Obesity’. New England Journal of Medicine 384, no. 11 (18 March 2021): 989–1002.
https://doi.org/10.1056/NEJMoa2032183.
STEP 2 - Davies et al.
Davies, Melanie, Louise Færch, Ole K Jeppesen, Arash Pakseresht, Sue D Pedersen, Leigh Perreault, Julio Rosenstock, et al. ‘Semaglutide 2·4 Mg Once a Week in Adults with Overweight or Obesity, and Type 2 Diabetes (STEP 2): A Randomised, Double-Blind, Double-Dummy, Placebo-Controlled, Phase 3 Trial’. The Lancet 397, no. 10278 (March 2021): 971–84.
https://doi.org/10.1016/S0140-6736(21)00213-0.
SUSTAIN 1 - Sorli et al.
Sorli, Christopher, Shin-ichi Harashima, George M Tsoukas, Jeffrey Unger, Julie Derving Karsbøl, Thomas Hansen, and Stephen C Bain. ‘Efficacy and Safety of Once-Weekly Semaglutide Monotherapy versus Placebo in Patients with Type 2 Diabetes (SUSTAIN 1): A Double-Blind, Randomised, Placebo-Controlled, Parallel-Group, Multinational, Multicentre Phase 3a Trial’. The Lancet Diabetes & Endocrinology 5, no. 4 (April 2017): 251–60.
https://doi.org/10.1016/S2213-8587(17)30013-X.
SUSTAIN 2 - Ahren et al.
Ahrén, Bo, Luis Masmiquel, Harish Kumar, Mehmet Sargin, Julie Derving Karsbøl, Sanja Hald Jacobsen, and Francis Chow. ‘Efficacy and Safety of Once-Weekly Semaglutide versus Once-Daily Sitagliptin as an Add-on to Metformin, Thiazolidinediones, or Both, in Patients with Type 2 Diabetes (SUSTAIN 2): A 56-Week, Double-Blind, Phase 3a, Randomised Trial’. The Lancet Diabetes & Endocrinology 5, no. 5 (May 2017): 341–54.
https://doi.org/10.1016/S2213-8587(17)30092-X.
FORTE - Frias et al.
Frías, Juan P, Pernille Auerbach, Harpreet S Bajaj, Yasushi Fukushima, Ildiko Lingvay, Stanislava Macura, Anette L Søndergaard, Tsvetalina I Tankova, Nikolaos Tentolouris, and John B Buse. ‘Efficacy and Safety of Once-Weekly Semaglutide 2·0 Mg versus 1·0 Mg in Patients with Type 2 Diabetes (SUSTAIN FORTE): A Double-Blind, Randomised, Phase 3B Trial’. The Lancet Diabetes & Endocrinology 9, no. 9 (September 2021): 563–74.
https://doi.org/10.1016/S2213-8587(21)00174-1.